|
|
|
|
Vitamin E: What It Is And What It Does
What is vitamin E? Vitamin E is a generic term that includes all entities that exhibit
the biological activity of alpha-tocopherol. 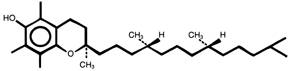 d-alpha-tocopherol In nature, eight substances have been found to have vitamin E
activity: alpha-, beta-, gamma- and delta-tocopherol; and alpha-, beta-,
gamma- and delta-tocotrienol. The acetate and succinate derivatives of
the natural tocopherols have vitamin E activity, as do synthetic
tocopherols and their acetate and succinate derivatives. Of these, d-alpha-tocopherol (RRR-alpha-tocopherol) has the highest
bioavailability and is the standard against which all the others must be
compared.
Is there a difference between natural and synthetic vitamin E? Natural and synthetic vitamin E are not equivalent in composition,
structure or bioavailability. Natural vitamin E (RRR-alpha-tocopherol or
d-alpha-tocopherol) is a single entity.  Synthetic vitamin E (all-rac-alpha-tocopherol or dl-alpha-tocopherol) is a mixture of eight stereoisomers in equal amounts. The other seven stereoisomers have different molecular configurations and lower biological activities that range from 21-90% of the activity of natural vitamin E based on rat assays. Natural vitamin E is officially recognized as having 36% greater potency than its synthetic counterpart as determined by studies in animals. However, recent human studies using deuterium-labeled vitamin E indicate that the bioavailability of natural vitamin E is approximately twice that of synthetic vitamin E, as natural vitamin E is retained longer in body tissues. In a recent study of six healthy volunteers, the ratio of labeled natural vitamin E in blood plasma was 2.1 times higher than that of synthetic vitamin E after 24 hours. In contrast, the level of a breakdown product of vitamin E in urine was about 2.7 times higher from synthetic vitamin E.1 In another study of six healthy subjects, supplementation with natural vitamin E resulted in two-fold higher blood plasma vitamin E levels than synthetic vitamin E.2 The bioavailability of natural vitamin E administered at 100 mg per day was similar to that of synthetic vitamin E acetate administered at 300 mg per day in a study of seven healthy women.3 Results of a study of 15 pregnant women supplemented with natural and synthetic vitamin E acetate showed that the average ratio of natural to synthetic vitamin E was 1.86 in maternal blood and 3.42 in cord blood.4 In healthy volunteers, the ratio of natural to synthetic vitamin E in blood plasma ranged from 1.5 to 1.8 during supplementation, with equal doses of both vitamin E compounds, and increased to 2.0 after supplementation ended. For elective surgery patients, the ratio of natural to synthetic vitamin E was about 1.7 in blood plasma and 1.5 in all tissues except the liver. The ratio of natural to synthetic vitamin E was 2.0-2.1 in blood plasma and 1.7-2.0 in tissues of two terminally ill patients supplemented with vitamin E for 1-2 years.5 Lower retention of synthetic vitamin E should be anticipated as there are specific protein receptors for d-alpha-tocopherol that make its transport and retention more efficient.6 The alpha-tocopherol transfer protein, which is present in the liver, binds vitamin E and enhances transport of the vitamin between membranes. This transfer protein binds alpha-tocopherol in preference to other homologs (beta-, gamma- and delta-), and the naturally occurring stereoisomer (RRR-alpha-tocopherol or d-alpha-tocopherol) in preference to the other stereoisomers present in synthetic vitamin E.7 1. Traber, M.G., Elsner, A.
and Brigelius-Flohe, R. Synthetic as Compared with Natural Vitamin E Is
Preferentially Excreted as Alpha-CEHC in Human Urine: Studies Using
Deuterated Alpha-Tocopheryl Acetates. FEBS Letters 437:145-148,
1998.
2. Acuff, R.V., Thedford, S.S., Hidiroglou, N.N.,
Papas, A.M. and Odom, T.A. Relative Bioavailability of RRR- and All-Rac-Alpha-Tocopheryl
Acetate in Humans: Studies Using Deuterated Compounds. Am. J. Clin.
Nutr. 60:397-402, 1994.
3. Kiyose, C., Muramatsu, R., Kameyama, Y.,
Ueda, T. and Igarashi, O. Biodiscrimination of Alpha-Tocopherol
Stereoisomers in Humans after Oral Administration. Am. J. Clin. Nutr.
65:785-789, 1997.
4. Acuff, R.V., Dunworth, R.G., Webb, L.W. and
Lane, J.R. Transport of Deuterium-Labeled Tocopherols during Pregnancy. Am.
J. Clin. Nutr. 67:459-464, 1998.
5. Burton, G.W., Traber, M.G., Acuff, R.V.,
Walters, D.N., Kayden, H., Hughes, L., and Ingold, K.U. Humana Plasma
and Tissue Alpha-Tocopherol Concentrations in Response to
Supplementation with Deuterated Natural and Synthetic Vitamin E. Am.
J. Clin. Nutr. 67:669-684, 1998.
6. Kitabchi, A.E., Wimalasena, J. and
Barker, J. Specific Receptor Sites for Alpha-Tocopherol in Purified
Isolated Adrenocortical Cell Membrane. Biochem. Biophys. Res. Comm.
96:1739-1746, 1980.
7. Hosomi, A., Arita, M., Sato, Y., Kiyose,
C., Ueda, T., Igarashi, O., Arai, H., and Inoue, K. Affinity for Alpha-Tocopherol
Transfer Protein as a Determinant of the Biological Activities of
Vitamin E Analogs. FEBS Letters. 409:105-108, 1997.
Vitamin E functions in several ways: The first, and most important, is as an antioxidant that protects cells and other body components from free radical attack. Oxidative damage resulting from free radical attack has been linked to the onset of premature aging, cancer, coronary heart disease, cataracts and an array of degenerative diseases. Vitamin E stimulates the immune response. Some studies have shown lower incidence of infections when vitamin E levels are high, and vitamin E may inhibit cancer initiation through enhanced immunocompetence. Vitamin E appears to lessen the severity of prostaglandin-mediated disorders such as inflammation, premenstrual syndrome and circulatory irregularities (nocturnal leg cramps and blood platelet adhesion). Vitamin E also has a direct chemical function. It inhibits the conversion of nitrites in smoked, pickled and cured foods to nitrosamines in the stomach. Nitrosamines are strong tumor promoters. Vitamin E has a regulatory effect on gene expression. It has been
shown to control proliferation of smooth muscle cells, which are
involved in the development of atherosclerosis.
How does vitamin E
function as an antioxidant? Vitamin E, which is fat soluble, is present in lipids, most significantly in the lipids of cell membranes and in circulating low-density lipoproteins. Free radicals produced as byproducts of metabolic processes and those originating from environmental pollutants (such as nitrogen dioxide and ozone of polluted air, heavy metals, halogenated hydrocarbons, ionizing radiation and cigarette smoke) are scavenged by available vitamin E. Unchecked by an antioxidant, the highly unstable free radicals attack
cell constituents, including DNA and other opportune targets,
particularly those containing polyunsaturated fatty acids (PUFAs). When
free radicals react with PUFAs, chain reactions generate free radicals
in profusion. Free radicals can damage both the structure and function
of cell membranes; nucleic acids and electron-dense regions of proteins
also come under attack. This can result in:
Is vitamin E a "typical"
vitamin? Vitamin E is more appropriately described as an antioxidant than a vitamin. Most vitamins function as cofactors for enzymatic reactions. Vitamin E does not function as a cofactor. Requirements for and utilization of vitamin E vary according to an individual's oxidative stress status. Concentrations of free radicals, other oxidants and the polyunsaturated fatty acid content of body tissues are major determinants of oxidative stress status. Also, deficiency of vitamin E does not produce a disease with rapidly
developing symptoms such as scurvy, beriberi, pellagra, rickets or
xerophthalmia. In humans, vitamin E produces overt deficiency symptoms
in cases involving fat malabsorption syndromes, premature infants and
patients on total parenteral nutrition. The effects of inadequate
vitamin E intake usually develop over a long time, typically decades,
and have been linked to degenerative diseases such as cancer,
atherosclerosis and other forms of heart disease.
Clinical conditions in
which free radicals are thought to be involved Following is a listing of scores of conditions to which free radicals have been linked.1 Most likely, free radicals will be the sole cause of only a few. However, free radicals may predispose the human body to a disease that is directly caused by other factors–that is, they may make some conditions worse–and may be an antagonist to the body's natural healing processes. In these cases, vitamin E would be useful as an adjunct therapy.
Free radical-related conditions: Primary single organ involvement Heart and cardiovascular system
Brain
Lung
Skin
Eye
Joint abnormalities
Gastrointestinal tract
Kidney
Erythrocytes
Multiorgan involvement Cancer Aging
Inflammatory-immune injury
Ischemia-reflow states Drug and toxin-induced reactions Iron overload
Nutritional deficiencies
Alcohol damage Radiation injury Amyloid diseases
1. Cross, C.E. Oxygen Radicals and Human Disease. Ann. Intern. Med. 107:526-545, 1987. |